July 2025: Is Your Coagulation Laboratory Inspection Ready?
by Donna Castellone • July 02, 2025

INTRODUCTION:
One of the biggest questions I get asked is how to handle coagulation laboratories and be inspection ready. People always ask when I cite a directive, well if I have never done this before, and not been cited, why should I do it now? Inspections occur periodically, however, adhering to the regulations and requirements are part of a quality system that should always exist. Having a balanced approach to quality can improve processes.
Just the thought of a laboratory inspection can make even the most seasoned technologist quake in their boots! It brings anxiety, pressure and stress in an already stressful work environment. Striving for compliance with regulatory and accreditation standards may seem unclear or redundant, however the checklists and requirements are your framework for helping to alleviate anxiety.1
Inspections verify that the laboratory has met the standards established by the accrediting agency. The outcome of all agencies is the goal to improve quality of care.2 However one of the biggest problems is that most laboratories are overseen by several agencies, regulations may overlap or differ. The best way to handle this is to adhere to the strictest recommendation so you are in compliance with all of them. Laboratory accreditation programs review processes and personnel. Several agencies include: CLIA: Clinical Laboratory Improvement Amendments, CAP: College of American Pathologists, ISO: International Organization for Standardization, and JC: Joint Commission are the most common.2 It is important to also be aware of individual state inspection requirements. Most laboratories are not inspected by numerous agencies due to deemed status which declare inspections are equivalent to those of a primary agency and do not require to be dually inspected.
Options for lab accrediation
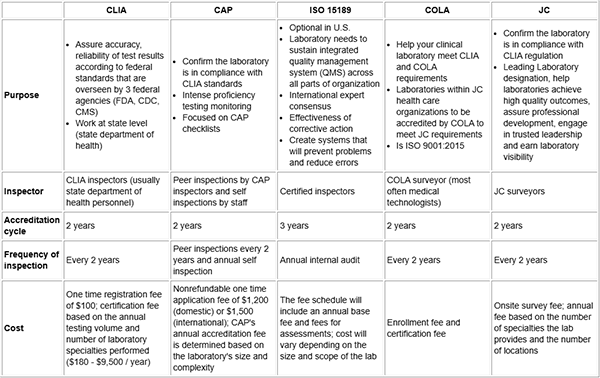
Adapted from: Tran MT, Hassell LA. Laboratory inspection and accreditation2
Deficiencies are identified during the inspection process that do not align with the required directive and need the attention of the laboratory. There are two types. Phase 1 deficiencies need to be addressed and may require long term improvements but do not pose an imminent risk to patient care or quality. Phase 2 deficiencies are items that must be corrected prior to receiving accreditation2
Most common laboratory deficiencies across accrediting agencies:
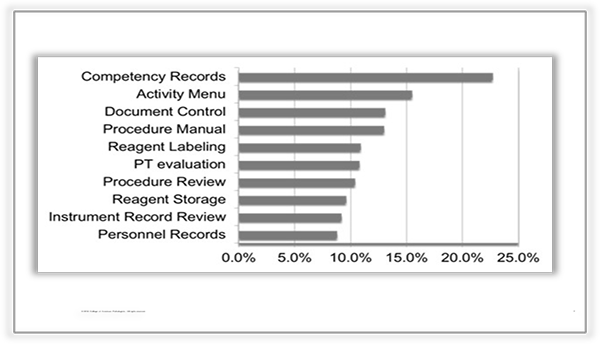
The most common deficiencies revolve around records, personnel and reagents. But what about the coagulation laboratory and deficiencies? Coagulation is complex enough and difficult to understand, what should your laboratory be doing to always be inspection ready?
INSPECTING THE COAGULATION LABORATORY:
One of the most common issues that is confusing to laboratories is: How do I make sure my coagulation analyzer is in compliance from validation, to correlation, to lot to lot validation? How many samples do I use?
What are you providing to your clinicians with your coagulation results? Information they will use to diagnosis and treat coagulation disorders. What is the risk your laboratory is willing to take- results that may be sufficient, results that may be questionable or results that truly reflect physiological occurrences? With this perspective in mind, your director should support the process for validating your testing.
First, determine your strictest regulations, and what the manufacturer requires and look at your base level requirements. For a full validation for a new analyzer, guidelines recommend 120 samples which is overwhelming for many laboratories. This will give you 99% confidence levels. Using a lower number will change your confidence level. Is your laboratory willing to accept a 95% confidence level? If you are looking to verify your anayzer you can use as low as 20 samples. Again, make sure your directives in your regulatory agency align with this process and your laboratory accepts the risk. The same hold true for establishing reference ranges. If your laboratory chooses to verify a manufacturer's range or a published range you can do so with 20 normal samples, as long as the normal sample that were used in the initial range match the patient population you are testing. For example, you do not want to verify a reference range that was established using an exclusively Asian population if this is not the majority of patients you are testing. It always helps to have published data supporting numbers, as well as any guidance from the regulatory agencies.
What about instrument correlation? Every 6 months, how many samples? Again, you can use a minimum of 20 samples, as long as they represent good samples across the reportable range. For some of the more expensive and infrequently used tests, 10 well classified samples (abnormal, borderline, normal and high) with good acceptability limits should reflect how your analyzers are correlating. There should be practices in place that state if the correlation is not acceptable, that additional samples are required. So what is an acceptable limit? Look again at the amount of risk you are willing to take, and what will demonstrate clinical significance. Sometimes, results are statistically significant, but not clinically significant. You can look at package inserts or clinical guidelines as well as what your director feels is acceptable and adhere to that limit.
One of the most important things to remember is that your policies, must reflect what you are actually doing, that means practice what you preach!! If your acceptability limit is 10% for an analyte that should be stated, another may be 20%, while another may have CLIA regulations for acceptability, that should be your guidance. When being inspected, this is something that should be looked at and be traceable back to your validation and or verification documents. Again, all signed off by the director as the amount of risk the laboratory is willing to take with this testing.
So we have, validation, correlation, and reference ranges. The next step is precision. It is so important for this to determine the CVs for your test, and how your analyzer is performing in BOTH the normal and abnormal range. I am not a fan of using controls to perform this, since they are adulterated and buffered samples. I prefer to use patient pools that reflect your patient matrix, and also have separate samples, meaning the probe from the analyzer doesn’t go into the same tube 10 times- but has to sample 10 tubes. This then mimics how the analyzer runs samples and better represents how samples are tested. This should be performed for normal and abnormal samples. It also with some testing to have samples at the borderline, to demonstrate how your analyzer performs in that range- so does a Protein C of 65 become 72, or is it the same result?
What about lot-to-lot validation? The same rules apply as to validation of a new analyzer, what is the risk you are willing to take? A new lot of PT/aPTT reagents should not be taken lightly. I always hear, well I don't do any specialized testing, only routine, well that is the most important testing done, it is the basis for special testing. Sample number again should look at the risk the laboratory is willing to take. Additionally, remember the specifics of testing MUST be written in your procedures. For example, when changing aPTT reagents for a Factor VIII assay, we may use 20 well classified samples across the reportable range, and acceptability must fall within stated criteria. However, when changing a component, say a new factor deficient plasma with the same aPTT reagent, you may choose to run controls and just a few (2-5) samples of normal and abnormal patients to ensure that the deficient plasma will not impact results. For shipment to shipment, a minimum of controls and a normal and abnormal patient should be checked. Again, all of this should be reflected in your procedures for these processes.
References that may help you include:
Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis
https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.16776?msockid=01e56ca7a40a674a2e307f25a5806637
---
CLSI H47: One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test, 3rd Edition
CLSI H48: Determination of Coagulation Factor Activities Using the One-Stage Clotting Assay, 2nd Edition
To Access for purchase: https://clsi.org/standards/products/hematology/documents/?page=2&sort=FriendlyCode&sortdir=asc&subcat=&area=Documents
---
ICSH, the International Council for Standardization in Haematology is pleased to announce the Publication of a new ICSH Guideline for New Lot Verification of Coagulation Reagents, Calibrators, and Controls
https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0043-1776405.pdf
PROFICIENCY SAMPLES
How do you handle when you fail a proficiency sample? When we do proficiency samples we do several things to make investigations a little less overwhelming. When the samples are run, we make sure to print copies of controls, as well as the standard curve that was used for testing, and the patient samples that were run concurrently with the PT samples. This helps to determine if possibly controls were running slightly higher, or lower, or if possibly a point was slightly different in particular in the reporting range of the sample. We also save patient samples that were run with the PT sample to determine if there was a failure if that impacted the patients' result or more likely random error. Maintenance records are easy to retrieve so we do not print them. Rerunning a PT sample is not the solution to solving this problem, it is part of the investigation. Having all of these tools on hand makes investigations easier and can support if it is a reagent error, instrument error or a random error? There needs to be a complete investigation and report, and corrective action documented and signed by the director. Investigation of PT failures are some of the most common deficiencies that occur.
If you test a sample that is not part of a proficiency testing program, you must make sure that the laboratory has a documented protocol for determining that your test is correct. The incidence of this error occurring in laboratories was at 2% in 2020 and has increased to 17% in 2021. Laboratories need to verify the analytes' accuracy twice annually.3 The easiest way to change this is to find a PT program that includes this particular test. If that is not possible, you can do a few things. You can split blind samples and have them tested internally by 2 different technologists and compare results. You can have a director compare the laboratory sample results to the clinical picture of a patient, which is signed off by the director with a report, or samples can be sent to another laboratory that performs the same test and results can be compared.
HOW TO STAY INSPECTION READY:
The initial preparation to arrange this may seem overwhelming, but in the long run it will make your laboratory ready and remove some of the anxiety that occurs with inspections.
- Establish a base line: Conduct an internal audit, you can either use the inspecting agencies checklist or perform a tracer audit for a specific test. I recommend both. A checklist provides an overview of testing, while a tracer audit can investigate deeper issues with testing that may be missed, pre-analytical, analytical and post analytical.
- Make sure that you have organized records, as well as the initial validation of your analyzer. It is difficult at times because you may take over a position and you may not be able to find initial validation records. Every effort should be made to find those records, if not possible, ensure that your current verification records are completed. It is very important that you are as organized as possible it really makes your life and the inspectors' job much easier and less prone to looking deeper into processes.
- Engage your staff: make sure that everyone is involved, assigned people to check expiration dates, following a checklist. Someone is responsible for weekly checks on analyzer maintenance, inventory logs and temperature checks. This is one of the highest deficiencies in inspections.
- Have a calendar that looks at when correlations are due, as well as PT testing, and how it should be rotated with staff. Have staff prepare for correlations, and use it as part of competency assessment.
- When filing PT challenges, make sure that they are signed off by directors and that proper investigations have been conducted. All testing must have either a formal PT program or an alternative method of ensuring accuracy.
- Make sure that your procedures match what you are doing. This includes on line manuals the laboratory makes available for clinicians. In particular if reference ranges change due to new analyzers or reagents. All downstream systems must match what is correct. I strongly suggest that either the laboratory or your IT department document that all systems, manuals, etc. match.
Having all of these systems in place can help your coagulation laboratory be inspection ready 24/7!
REFERENCES:
- Essential steps for laboratory inspections
https://montfortlabs.com/education/essential-steps-successful-laboratory-inspections/ - Tran MT, Hassell LA. Laboratory inspection and accreditation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/labadmininspectionaccreditation.html. Accessed June 27th, 2025. https://www.pathologyoutlines.com/topic/labadmininspectionaccreditation.html
- Top Laboratories Deficiencies of 2020-2021
https://achc.org/wp-content/uploads/2022/04/top-laboratory-deficiencies.pdf
